
Datopotamab Deruxtecan (DATROWAY): A Paradigm Shift in Metastatic Breast Cancer Treatment

Datopotamab Deruxtecan (DATROWAY): A Scientific Breakthrough in Targeted Cancer Therapy
Introduction
The field of oncology has undergone a transformative shift with the advent of targeted therapies, which have substantially improved the precision and efficacy of cancer treatments. Datopotamab Deruxtecan (DATROWAY), a cutting-edge antibody-drug conjugate (ADC), has emerged as a pioneering intervention in the therapeutic arsenal against metastatic breast cancer. This article provides a comprehensive exploration of its intricate mechanisms of action, landmark clinical trials, comparative efficacy with existing treatment modalities, safety considerations, and potential future applications in a broader oncology spectrum.
Mechanism of Action: A Synergistic Approach to Tumor Targeting
Datopotamab Deruxtecan is a next-generation TROP2-directed ADC designed to selectively deliver cytotoxic agents to malignant cells, thus minimizing off-target effects and enhancing therapeutic efficacy. The ADC is composed of:
- Monoclonal Antibody (mAb) Targeting TROP2: This component confers high specificity by binding to the TROP2 transmembrane glycoprotein, which is aberrantly overexpressed in multiple solid tumors, particularly metastatic breast cancer.
- Deruxtecan Payload: A potent topoisomerase I inhibitor that exerts its cytotoxic effects following ADC internalization and intracellular release, leading to DNA damage and apoptotic cell death.
Pharmacodynamic and Pharmacokinetic Profile
- Upon engagement with TROP2-expressing tumor cells, the ADC undergoes receptor-mediated internalization.
- Intracellular enzymatic cleavage liberates the Deruxtecan payload, which diffuses into the nucleus and exerts its cytotoxic activity by inhibiting topoisomerase I.
- The inclusion of an optimized linker system ensures controlled payload release, thereby enhancing the drug’s therapeutic index while mitigating systemic toxicity.
Clinical Efficacy: Insights from the TROPION-Breast01 Trial
A pivotal phase 3 study, the TROPION-Breast01 trial, was conducted to assess the clinical efficacy and safety of Datopotamab Deruxtecan in patients with metastatic breast cancer who had demonstrated resistance to standard therapies.
- Study Design: A multicenter, randomized, controlled trial comparing DATROWAY to the physician’s choice of chemotherapy.
- Primary Endpoint: Progression-free survival (PFS), a key indicator of treatment efficacy.
- Secondary Endpoints: Overall survival (OS), objective response rate (ORR), duration of response (DOR), and safety profile.
- Key Findings:
- DATROWAY demonstrated a statistically significant improvement in PFS and OS, reinforcing its superiority over traditional chemotherapy.
- Enhanced rates of durable tumor regression were observed, suggesting prolonged therapeutic benefit.
- The toxicity profile was more favorable, with fewer incidences of severe adverse reactions compared to standard cytotoxic regimens.
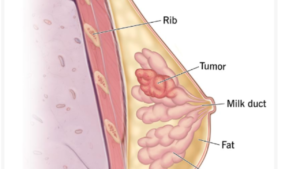
Comparative Analysis: DATROWAY vs. Existing Therapies
| Therapeutic Modality | Mechanism of Action | Clinical Benefit | Adverse Effects |
|---|---|---|---|
| Datopotamab Deruxtecan | TROP2-targeted ADC + Deruxtecan | High tumor response rates | Lower systemic toxicity |
| Durvalumab (PD-L1 Inhibitor) | Immune checkpoint inhibition | Effective in select patients | Immune-related toxicities |
| Traditional Chemotherapy | Non-specific cytotoxicity | Broad tumor shrinkage | Significant systemic toxicity |
| Sacituzumab Govitecan | TROP2-directed ADC | Effective in TNBC | Moderate toxicity risk |
Adverse Events and Safety Considerations
Although Datopotamab Deruxtecan exhibits a favorable therapeutic profile, it remains associated with certain adverse effects that necessitate vigilant monitoring:
- Common: Fatigue, nausea, myelosuppression, diarrhea.
- Serious but Rare: Interstitial lung disease (ILD), necessitating early intervention to mitigate morbidity and mortality.
Expanding Therapeutic Horizons: Future Clinical Applications
Given its remarkable efficacy in metastatic breast cancer, Datopotamab Deruxtecan is currently under investigation for its potential application in other malignancies, including:
- Non-small cell lung cancer (NSCLC) – Clinical trials are evaluating its impact on TROP2-expressing lung tumors.
- Triple-negative breast cancer (TNBC) – Potential as a frontline therapy due to its targeted cytotoxicity.
- Bladder carcinoma – Studies suggest that TROP2 expression in urothelial carcinoma may render it susceptible to ADC-based therapies.
Additionally, emerging research is exploring synergistic combination therapies, particularly with immune checkpoint inhibitors, to optimize response rates and extend the durability of clinical benefit.
Frequently Asked Questions (FAQs)
1. Has Datopotamab Deruxtecan received FDA approval?
Currently in late-stage clinical evaluation, regulatory approval is anticipated based on strong efficacy and safety data from ongoing trials.
2. How does DATROWAY compare to conventional chemotherapy?
Its targeted mechanism allows for higher efficacy with significantly reduced collateral damage to normal tissues, thereby improving tolerability.
3. Can DATROWAY be used in combination with other therapies?
Combination strategies, particularly with immune checkpoint inhibitors such as Durvalumab, are under active clinical investigation.
4. What patient population is eligible for DATROWAY therapy?
Patients with metastatic breast cancer exhibiting TROP2 overexpression who have exhausted prior treatment options may be suitable candidates.
Conclusion
Datopotamab Deruxtecan (DATROWAY) represents a monumental advancement in targeted cancer therapy, demonstrating unparalleled efficacy and a favorable safety profile in metastatic breast cancer. As research continues to expand its applications across multiple malignancies and combination strategies, DATROWAY is poised to redefine the landscape of precision oncology, offering renewed hope for patients with advanced-stage cancer.



Comments